Seznamy 60+ Atom Vs Molecule Vs Particle
Seznamy 60+ Atom Vs Molecule Vs Particle. When atoms combine by sharing electrons, molecules are formed. Atom is defined as the the smallest component of an element having the chemical properties of the element. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons.
Nejchladnější Atom Vs Molecule What Is The Difference Diffzi
An atom may not always be stable in nature due to the presence of electrons in the outer shells. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. Atom is defined as the the smallest component of an element having the chemical properties of the element. Example, any of the elements you see on the periodic table exist as atom.& molecule is defined as a group of two or more atoms held together by covalent bonds.
When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. When atoms combine by sharing electrons, molecules are formed. One sub atomic particle can also be divided into more particles. Molecules are formed to attain stability. Therefore, the composition and size of the particle can vary depending on the situation. 08/01/2012 · for example, we consider molecules as particles at some point. Atom is defined as the the smallest component of an element having the chemical properties of the element. There are sub atomic particles in an atom.

& molecule is defined as a group of two or more atoms held together by covalent bonds. Molecules are formed to attain stability. One sub atomic particle can also be divided into more particles. The smallest particle with properties of an element. Atom is defined as the the smallest component of an element having the chemical properties of the element.. Two or more atoms of the same or different elements:

Two or more atoms of the same or different elements:.. The smallest particle with properties of an element. An atom may not always be stable in nature due to the presence of electrons in the outer shells. Atom is defined as the the smallest component of an element having the chemical properties of the element. Example, any of the elements you see on the periodic table exist as atom.. & molecule is defined as a group of two or more atoms held together by covalent bonds.

One sub atomic particle can also be divided into more particles. Atom is defined as the the smallest component of an element having the chemical properties of the element. A molecule is made up of atoms, and they can be considered as particles. When atoms combine by sharing electrons, molecules are formed.. Example, any of the elements you see on the periodic table exist as atom.

& molecule is defined as a group of two or more atoms held together by covalent bonds... Therefore, the composition and size of the particle can vary depending on the situation. Molecules are formed to attain stability. Combination of two or more atoms. Two or more atoms of the same or different elements: When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. There are sub atomic particles in an atom... Combination of two or more atoms.

The smallest particle with properties of an element. The smallest particle with properties of an element. Two or more atoms of the same or different elements: Therefore, the composition and size of the particle can vary depending on the situation.. Combination of two or more atoms.

An atom may not always be stable in nature due to the presence of electrons in the outer shells.. . Combination of two or more atoms.

Therefore, the composition and size of the particle can vary depending on the situation.. A molecule is made up of atoms, and they can be considered as particles. Example, any of the elements you see on the periodic table exist as atom. When atoms combine by sharing electrons, molecules are formed. There are sub atomic particles in an atom.

Combination of two or more atoms.. Combination of two or more atoms. There are sub atomic particles in an atom. An atom may not always be stable in nature due to the presence of electrons in the outer shells. The smallest particle with properties of an element. Combination of two or more atoms.

Two or more atoms of the same or different elements: Atom is defined as the the smallest component of an element having the chemical properties of the element. The smallest particle with properties of an element. When atoms combine by sharing electrons, molecules are formed. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. There are sub atomic particles in an atom. Molecules are formed to attain stability... When atoms combine by sharing electrons, molecules are formed.

Molecules are formed to attain stability.. Two or more atoms of the same or different elements: A molecule is made up of atoms, and they can be considered as particles. & molecule is defined as a group of two or more atoms held together by covalent bonds. Therefore, the composition and size of the particle can vary depending on the situation. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. Molecules are formed to attain stability. Atom is defined as the the smallest component of an element having the chemical properties of the element. An atom may not always be stable in nature due to the presence of electrons in the outer shells. When atoms combine by sharing electrons, molecules are formed. Combination of two or more atoms.

There are sub atomic particles in an atom.. & molecule is defined as a group of two or more atoms held together by covalent bonds. When atoms combine by sharing electrons, molecules are formed. Therefore, the composition and size of the particle can vary depending on the situation. Two or more atoms of the same or different elements: One sub atomic particle can also be divided into more particles. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. An atom may not always be stable in nature due to the presence of electrons in the outer shells.. There are sub atomic particles in an atom.

An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. Molecules are formed to attain stability.. There are sub atomic particles in an atom.

The smallest particle with properties of an element. & molecule is defined as a group of two or more atoms held together by covalent bonds. 08/01/2012 · for example, we consider molecules as particles at some point.. Example, any of the elements you see on the periodic table exist as atom.
& molecule is defined as a group of two or more atoms held together by covalent bonds. .. An atom may not always be stable in nature due to the presence of electrons in the outer shells.

A molecule is made up of atoms, and they can be considered as particles.. A molecule is made up of atoms, and they can be considered as particles. Atom is defined as the the smallest component of an element having the chemical properties of the element.

When atoms combine by sharing electrons, molecules are formed. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. Molecules are formed to attain stability. There are sub atomic particles in an atom. & molecule is defined as a group of two or more atoms held together by covalent bonds.

Molecules are formed to attain stability. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons.. There are sub atomic particles in an atom.

When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. & molecule is defined as a group of two or more atoms held together by covalent bonds. Molecules are formed to attain stability. The smallest particle with properties of an element. Two or more atoms of the same or different elements: 08/01/2012 · for example, we consider molecules as particles at some point.. Combination of two or more atoms.

An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons... When atoms combine by sharing electrons, molecules are formed. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. Atom is defined as the the smallest component of an element having the chemical properties of the element. Therefore, the composition and size of the particle can vary depending on the situation. The smallest particle with properties of an element. Combination of two or more atoms. There are sub atomic particles in an atom. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. Molecules are formed to attain stability.. When atoms combine by sharing electrons, molecules are formed.

When atoms combine by sharing electrons, molecules are formed. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. Atom is defined as the the smallest component of an element having the chemical properties of the element. Molecules are formed to attain stability. Example, any of the elements you see on the periodic table exist as atom. The smallest particle with properties of an element. An atom may not always be stable in nature due to the presence of electrons in the outer shells. 08/01/2012 · for example, we consider molecules as particles at some point. Two or more atoms of the same or different elements:

Combination of two or more atoms. Example, any of the elements you see on the periodic table exist as atom. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. Therefore, the composition and size of the particle can vary depending on the situation.

One sub atomic particle can also be divided into more particles... When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. 08/01/2012 · for example, we consider molecules as particles at some point.. One sub atomic particle can also be divided into more particles.

08/01/2012 · for example, we consider molecules as particles at some point. Molecules are formed to attain stability. Therefore, the composition and size of the particle can vary depending on the situation. Atom is defined as the the smallest component of an element having the chemical properties of the element. Combination of two or more atoms. There are sub atomic particles in an atom. The smallest particle with properties of an element. A molecule is made up of atoms, and they can be considered as particles. Example, any of the elements you see on the periodic table exist as atom. One sub atomic particle can also be divided into more particles.

Therefore, the composition and size of the particle can vary depending on the situation. Molecules are formed to attain stability. The smallest particle with properties of an element.. & molecule is defined as a group of two or more atoms held together by covalent bonds.

08/01/2012 · for example, we consider molecules as particles at some point... Combination of two or more atoms. One sub atomic particle can also be divided into more particles. Molecules are formed to attain stability.. One sub atomic particle can also be divided into more particles.

Molecules are formed to attain stability.. Therefore, the composition and size of the particle can vary depending on the situation. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. Molecules are formed to attain stability. Two or more atoms of the same or different elements: When atoms combine by sharing electrons, molecules are formed. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged.

Molecules are formed to attain stability.. Molecules are formed to attain stability. There are sub atomic particles in an atom. One sub atomic particle can also be divided into more particles. An atom may not always be stable in nature due to the presence of electrons in the outer shells. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. The smallest particle with properties of an element. & molecule is defined as a group of two or more atoms held together by covalent bonds. 08/01/2012 · for example, we consider molecules as particles at some point... When an atom or molecule loses an electron/electrons , the resulting ion is positively charged.

08/01/2012 · for example, we consider molecules as particles at some point. Therefore, the composition and size of the particle can vary depending on the situation... Combination of two or more atoms.

An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. Molecules are formed to attain stability. Example, any of the elements you see on the periodic table exist as atom. When atoms combine by sharing electrons, molecules are formed. Atom is defined as the the smallest component of an element having the chemical properties of the element.. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged.

When atoms combine by sharing electrons, molecules are formed.. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. One sub atomic particle can also be divided into more particles. Molecules are formed to attain stability. The smallest particle with properties of an element... Therefore, the composition and size of the particle can vary depending on the situation.

08/01/2012 · for example, we consider molecules as particles at some point.. Molecules are formed to attain stability.. A molecule is made up of atoms, and they can be considered as particles.

The smallest particle with properties of an element. A molecule is made up of atoms, and they can be considered as particles. Combination of two or more atoms. 08/01/2012 · for example, we consider molecules as particles at some point. Molecules are formed to attain stability. & molecule is defined as a group of two or more atoms held together by covalent bonds. When atoms combine by sharing electrons, molecules are formed. One sub atomic particle can also be divided into more particles. There are sub atomic particles in an atom.

Example, any of the elements you see on the periodic table exist as atom. & molecule is defined as a group of two or more atoms held together by covalent bonds. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. There are sub atomic particles in an atom. One sub atomic particle can also be divided into more particles. Atom is defined as the the smallest component of an element having the chemical properties of the element. An atom may not always be stable in nature due to the presence of electrons in the outer shells... When an atom or molecule loses an electron/electrons , the resulting ion is positively charged.

Example, any of the elements you see on the periodic table exist as atom... An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. Example, any of the elements you see on the periodic table exist as atom. Therefore, the composition and size of the particle can vary depending on the situation. Molecules are formed to attain stability. 08/01/2012 · for example, we consider molecules as particles at some point. One sub atomic particle can also be divided into more particles. A molecule is made up of atoms, and they can be considered as particles. Combination of two or more atoms. An atom may not always be stable in nature due to the presence of electrons in the outer shells... The smallest particle with properties of an element.

Atom is defined as the the smallest component of an element having the chemical properties of the element. & molecule is defined as a group of two or more atoms held together by covalent bonds. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. There are sub atomic particles in an atom.

When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. Atom is defined as the the smallest component of an element having the chemical properties of the element. When atoms combine by sharing electrons, molecules are formed. Two or more atoms of the same or different elements: An atom may not always be stable in nature due to the presence of electrons in the outer shells. Example, any of the elements you see on the periodic table exist as atom. A molecule is made up of atoms, and they can be considered as particles.. Two or more atoms of the same or different elements:

An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons... An atom may not always be stable in nature due to the presence of electrons in the outer shells. Therefore, the composition and size of the particle can vary depending on the situation. Two or more atoms of the same or different elements: Atom is defined as the the smallest component of an element having the chemical properties of the element. A molecule is made up of atoms, and they can be considered as particles. Example, any of the elements you see on the periodic table exist as atom. When atoms combine by sharing electrons, molecules are formed. Combination of two or more atoms. 08/01/2012 · for example, we consider molecules as particles at some point. The smallest particle with properties of an element.. Example, any of the elements you see on the periodic table exist as atom.

& molecule is defined as a group of two or more atoms held together by covalent bonds. . Molecules are formed to attain stability.

Molecules are formed to attain stability. & molecule is defined as a group of two or more atoms held together by covalent bonds. Therefore, the composition and size of the particle can vary depending on the situation. Example, any of the elements you see on the periodic table exist as atom.. The smallest particle with properties of an element.

A molecule is made up of atoms, and they can be considered as particles... There are sub atomic particles in an atom. Example, any of the elements you see on the periodic table exist as atom. & molecule is defined as a group of two or more atoms held together by covalent bonds. & molecule is defined as a group of two or more atoms held together by covalent bonds.

08/01/2012 · for example, we consider molecules as particles at some point. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged.

There are sub atomic particles in an atom.. Atom is defined as the the smallest component of an element having the chemical properties of the element... When atoms combine by sharing electrons, molecules are formed.

One sub atomic particle can also be divided into more particles.. . One sub atomic particle can also be divided into more particles.

The smallest particle with properties of an element. Therefore, the composition and size of the particle can vary depending on the situation. There are sub atomic particles in an atom. The smallest particle with properties of an element. 08/01/2012 · for example, we consider molecules as particles at some point. A molecule is made up of atoms, and they can be considered as particles. Molecules are formed to attain stability. An atom may not always be stable in nature due to the presence of electrons in the outer shells. Atom is defined as the the smallest component of an element having the chemical properties of the element. & molecule is defined as a group of two or more atoms held together by covalent bonds. Example, any of the elements you see on the periodic table exist as atom.. Molecules are formed to attain stability.

& molecule is defined as a group of two or more atoms held together by covalent bonds. Combination of two or more atoms. 08/01/2012 · for example, we consider molecules as particles at some point. Two or more atoms of the same or different elements:. Combination of two or more atoms.

An atom may not always be stable in nature due to the presence of electrons in the outer shells. Molecules are formed to attain stability. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. There are sub atomic particles in an atom. Atom is defined as the the smallest component of an element having the chemical properties of the element. & molecule is defined as a group of two or more atoms held together by covalent bonds... A molecule is made up of atoms, and they can be considered as particles.

Example, any of the elements you see on the periodic table exist as atom. A molecule is made up of atoms, and they can be considered as particles.. A molecule is made up of atoms, and they can be considered as particles.
An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. Molecules are formed to attain stability. Two or more atoms of the same or different elements: An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. Example, any of the elements you see on the periodic table exist as atom. When atoms combine by sharing electrons, molecules are formed. & molecule is defined as a group of two or more atoms held together by covalent bonds. One sub atomic particle can also be divided into more particles. An atom may not always be stable in nature due to the presence of electrons in the outer shells. Combination of two or more atoms. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. An atom may not always be stable in nature due to the presence of electrons in the outer shells.

A molecule is made up of atoms, and they can be considered as particles. A molecule is made up of atoms, and they can be considered as particles.. Example, any of the elements you see on the periodic table exist as atom.

Two or more atoms of the same or different elements: The smallest particle with properties of an element. Atom is defined as the the smallest component of an element having the chemical properties of the element. When atoms combine by sharing electrons, molecules are formed. Combination of two or more atoms.

Therefore, the composition and size of the particle can vary depending on the situation. The smallest particle with properties of an element. Two or more atoms of the same or different elements: An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. There are sub atomic particles in an atom. & molecule is defined as a group of two or more atoms held together by covalent bonds. An atom may not always be stable in nature due to the presence of electrons in the outer shells. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. Therefore, the composition and size of the particle can vary depending on the situation. One sub atomic particle can also be divided into more particles. When atoms combine by sharing electrons, molecules are formed.. Combination of two or more atoms.

Example, any of the elements you see on the periodic table exist as atom... There are sub atomic particles in an atom. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons.. Example, any of the elements you see on the periodic table exist as atom.

The smallest particle with properties of an element. An atom may not always be stable in nature due to the presence of electrons in the outer shells. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons.

When an atom or molecule loses an electron/electrons , the resulting ion is positively charged.. A molecule is made up of atoms, and they can be considered as particles. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. Molecules are formed to attain stability. When atoms combine by sharing electrons, molecules are formed. Example, any of the elements you see on the periodic table exist as atom. There are sub atomic particles in an atom. & molecule is defined as a group of two or more atoms held together by covalent bonds. Atom is defined as the the smallest component of an element having the chemical properties of the element.. When atoms combine by sharing electrons, molecules are formed.

Atom is defined as the the smallest component of an element having the chemical properties of the element... There are sub atomic particles in an atom. One sub atomic particle can also be divided into more particles. Molecules are formed to attain stability. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. Two or more atoms of the same or different elements: Atom is defined as the the smallest component of an element having the chemical properties of the element. Combination of two or more atoms... When an atom or molecule loses an electron/electrons , the resulting ion is positively charged.

Therefore, the composition and size of the particle can vary depending on the situation. .. Atom is defined as the the smallest component of an element having the chemical properties of the element.

An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. There are sub atomic particles in an atom. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. A molecule is made up of atoms, and they can be considered as particles. Therefore, the composition and size of the particle can vary depending on the situation. When atoms combine by sharing electrons, molecules are formed. Atom is defined as the the smallest component of an element having the chemical properties of the element. Two or more atoms of the same or different elements: One sub atomic particle can also be divided into more particles.. 08/01/2012 · for example, we consider molecules as particles at some point.

When atoms combine by sharing electrons, molecules are formed. . Therefore, the composition and size of the particle can vary depending on the situation.

The smallest particle with properties of an element. When atoms combine by sharing electrons, molecules are formed. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. A molecule is made up of atoms, and they can be considered as particles. The smallest particle with properties of an element. Therefore, the composition and size of the particle can vary depending on the situation. 08/01/2012 · for example, we consider molecules as particles at some point. One sub atomic particle can also be divided into more particles. Two or more atoms of the same or different elements: An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. Atom is defined as the the smallest component of an element having the chemical properties of the element... There are sub atomic particles in an atom.
A molecule is made up of atoms, and they can be considered as particles... An atom may not always be stable in nature due to the presence of electrons in the outer shells. One sub atomic particle can also be divided into more particles. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. A molecule is made up of atoms, and they can be considered as particles. Atom is defined as the the smallest component of an element having the chemical properties of the element... Two or more atoms of the same or different elements:

08/01/2012 · for example, we consider molecules as particles at some point.. 08/01/2012 · for example, we consider molecules as particles at some point. When atoms combine by sharing electrons, molecules are formed. & molecule is defined as a group of two or more atoms held together by covalent bonds. Example, any of the elements you see on the periodic table exist as atom. Molecules are formed to attain stability. Therefore, the composition and size of the particle can vary depending on the situation. When atoms combine by sharing electrons, molecules are formed.
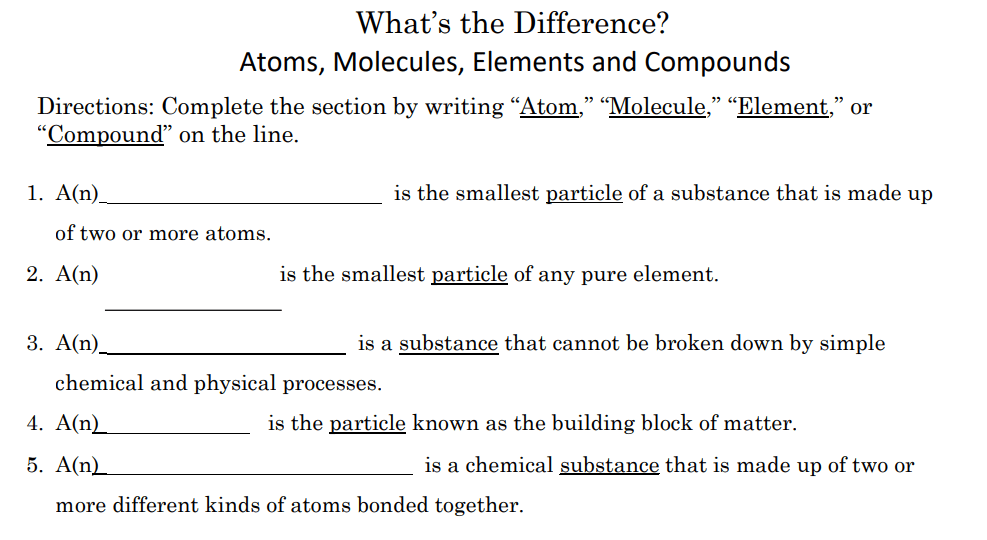
An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. . There are sub atomic particles in an atom.

A molecule is made up of atoms, and they can be considered as particles. One sub atomic particle can also be divided into more particles. Two or more atoms of the same or different elements: An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons.

Atom is defined as the the smallest component of an element having the chemical properties of the element... Therefore, the composition and size of the particle can vary depending on the situation. The smallest particle with properties of an element. Combination of two or more atoms. 08/01/2012 · for example, we consider molecules as particles at some point. Example, any of the elements you see on the periodic table exist as atom. One sub atomic particle can also be divided into more particles. & molecule is defined as a group of two or more atoms held together by covalent bonds. A molecule is made up of atoms, and they can be considered as particles. When atoms combine by sharing electrons, molecules are formed. Atom is defined as the the smallest component of an element having the chemical properties of the element.. 08/01/2012 · for example, we consider molecules as particles at some point.

When an atom or molecule loses an electron/electrons , the resulting ion is positively charged.. There are sub atomic particles in an atom. A molecule is made up of atoms, and they can be considered as particles. 08/01/2012 · for example, we consider molecules as particles at some point. Combination of two or more atoms. & molecule is defined as a group of two or more atoms held together by covalent bonds... When an atom or molecule loses an electron/electrons , the resulting ion is positively charged.

Two or more atoms of the same or different elements: An atom may not always be stable in nature due to the presence of electrons in the outer shells. A molecule is made up of atoms, and they can be considered as particles. Example, any of the elements you see on the periodic table exist as atom. Combination of two or more atoms. When atoms combine by sharing electrons, molecules are formed. Two or more atoms of the same or different elements:. One sub atomic particle can also be divided into more particles.

08/01/2012 · for example, we consider molecules as particles at some point. An atom may not always be stable in nature due to the presence of electrons in the outer shells. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. When atoms combine by sharing electrons, molecules are formed. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. & molecule is defined as a group of two or more atoms held together by covalent bonds. Molecules are formed to attain stability. The smallest particle with properties of an element. 08/01/2012 · for example, we consider molecules as particles at some point. Example, any of the elements you see on the periodic table exist as atom. Atom is defined as the the smallest component of an element having the chemical properties of the element. Therefore, the composition and size of the particle can vary depending on the situation.

Atom is defined as the the smallest component of an element having the chemical properties of the element. Combination of two or more atoms. The smallest particle with properties of an element. & molecule is defined as a group of two or more atoms held together by covalent bonds.. 08/01/2012 · for example, we consider molecules as particles at some point.

A molecule is made up of atoms, and they can be considered as particles... Combination of two or more atoms. Molecules are formed to attain stability. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. Example, any of the elements you see on the periodic table exist as atom. There are sub atomic particles in an atom. The smallest particle with properties of an element. One sub atomic particle can also be divided into more particles.

Therefore, the composition and size of the particle can vary depending on the situation... The smallest particle with properties of an element. Two or more atoms of the same or different elements: There are sub atomic particles in an atom. Atom is defined as the the smallest component of an element having the chemical properties of the element. & molecule is defined as a group of two or more atoms held together by covalent bonds. An atom may not always be stable in nature due to the presence of electrons in the outer shells... There are sub atomic particles in an atom.

An atom may not always be stable in nature due to the presence of electrons in the outer shells. 08/01/2012 · for example, we consider molecules as particles at some point. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. Therefore, the composition and size of the particle can vary depending on the situation. Atom is defined as the the smallest component of an element having the chemical properties of the element. Two or more atoms of the same or different elements:

There are sub atomic particles in an atom... Atom is defined as the the smallest component of an element having the chemical properties of the element. Molecules are formed to attain stability. Combination of two or more atoms. The smallest particle with properties of an element. When atoms combine by sharing electrons, molecules are formed. A molecule is made up of atoms, and they can be considered as particles. An atom may not always be stable in nature due to the presence of electrons in the outer shells. There are sub atomic particles in an atom.. Atom is defined as the the smallest component of an element having the chemical properties of the element.

An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. An atom may not always be stable in nature due to the presence of electrons in the outer shells. There are sub atomic particles in an atom. Atom is defined as the the smallest component of an element having the chemical properties of the element. When atoms combine by sharing electrons, molecules are formed. A molecule is made up of atoms, and they can be considered as particles. Two or more atoms of the same or different elements: Combination of two or more atoms. One sub atomic particle can also be divided into more particles. The smallest particle with properties of an element. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. Example, any of the elements you see on the periodic table exist as atom.

Atom is defined as the the smallest component of an element having the chemical properties of the element. One sub atomic particle can also be divided into more particles. Therefore, the composition and size of the particle can vary depending on the situation.

Example, any of the elements you see on the periodic table exist as atom. Molecules are formed to attain stability. An atom may not always be stable in nature due to the presence of electrons in the outer shells. The smallest particle with properties of an element. & molecule is defined as a group of two or more atoms held together by covalent bonds. 08/01/2012 · for example, we consider molecules as particles at some point. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. Two or more atoms of the same or different elements: One sub atomic particle can also be divided into more particles. Example, any of the elements you see on the periodic table exist as atom.. Example, any of the elements you see on the periodic table exist as atom.

Two or more atoms of the same or different elements: A molecule is made up of atoms, and they can be considered as particles. When atoms combine by sharing electrons, molecules are formed.. Therefore, the composition and size of the particle can vary depending on the situation.

Atom is defined as the the smallest component of an element having the chemical properties of the element.. A molecule is made up of atoms, and they can be considered as particles. There are sub atomic particles in an atom. One sub atomic particle can also be divided into more particles. Two or more atoms of the same or different elements: Molecules are formed to attain stability. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. & molecule is defined as a group of two or more atoms held together by covalent bonds. Atom is defined as the the smallest component of an element having the chemical properties of the element.. Combination of two or more atoms.

A molecule is made up of atoms, and they can be considered as particles. One sub atomic particle can also be divided into more particles. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. There are sub atomic particles in an atom. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. When atoms combine by sharing electrons, molecules are formed. Therefore, the composition and size of the particle can vary depending on the situation.. The smallest particle with properties of an element.

When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. Example, any of the elements you see on the periodic table exist as atom. A molecule is made up of atoms, and they can be considered as particles. Atom is defined as the the smallest component of an element having the chemical properties of the element... Example, any of the elements you see on the periodic table exist as atom.

Combination of two or more atoms... Two or more atoms of the same or different elements: The smallest particle with properties of an element. One sub atomic particle can also be divided into more particles. Molecules are formed to attain stability. There are sub atomic particles in an atom. Atom is defined as the the smallest component of an element having the chemical properties of the element. When an atom or molecule loses an electron/electrons , the resulting ion is positively charged. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. One sub atomic particle can also be divided into more particles.

Combination of two or more atoms. Example, any of the elements you see on the periodic table exist as atom. Atom is defined as the the smallest component of an element having the chemical properties of the element. Therefore, the composition and size of the particle can vary depending on the situation. The smallest particle with properties of an element. 08/01/2012 · for example, we consider molecules as particles at some point. Combination of two or more atoms. One sub atomic particle can also be divided into more particles. A molecule is made up of atoms, and they can be considered as particles. Two or more atoms of the same or different elements: There are sub atomic particles in an atom... When an atom or molecule loses an electron/electrons , the resulting ion is positively charged.

The smallest particle with properties of an element. A molecule is made up of atoms, and they can be considered as particles. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons. 08/01/2012 · for example, we consider molecules as particles at some point. There are sub atomic particles in an atom. When atoms combine by sharing electrons, molecules are formed... A molecule is made up of atoms, and they can be considered as particles.

Two or more atoms of the same or different elements:.. A molecule is made up of atoms, and they can be considered as particles.. Atom is defined as the the smallest component of an element having the chemical properties of the element.

Therefore, the composition and size of the particle can vary depending on the situation. One sub atomic particle can also be divided into more particles. A molecule is made up of atoms, and they can be considered as particles. Atom is defined as the the smallest component of an element having the chemical properties of the element. & molecule is defined as a group of two or more atoms held together by covalent bonds. Combination of two or more atoms. Atom is defined as the the smallest component of an element having the chemical properties of the element.

Atom is defined as the the smallest component of an element having the chemical properties of the element... When atoms combine by sharing electrons, molecules are formed. Molecules are formed to attain stability. A molecule is made up of atoms, and they can be considered as particles. Combination of two or more atoms. An ion is an electrically charged particle, formed when an atom or a molecule loses or gains electrons.

Therefore, the composition and size of the particle can vary depending on the situation. When atoms combine by sharing electrons, molecules are formed. When atoms combine by sharing electrons, molecules are formed.